Prescribe A
New Reality
Discover how the clinically validated RelieVRx® program can relieve pain intensity and interference for chronic lower back pain (CLBP) without the burden and potential risks of other common modalities.1,2
Prescribe Nowthe first FDA-authorized Virtual Reality (VR) treatment for CLBP1
A single course of treatment delivers lasting relief of CLBP2,3
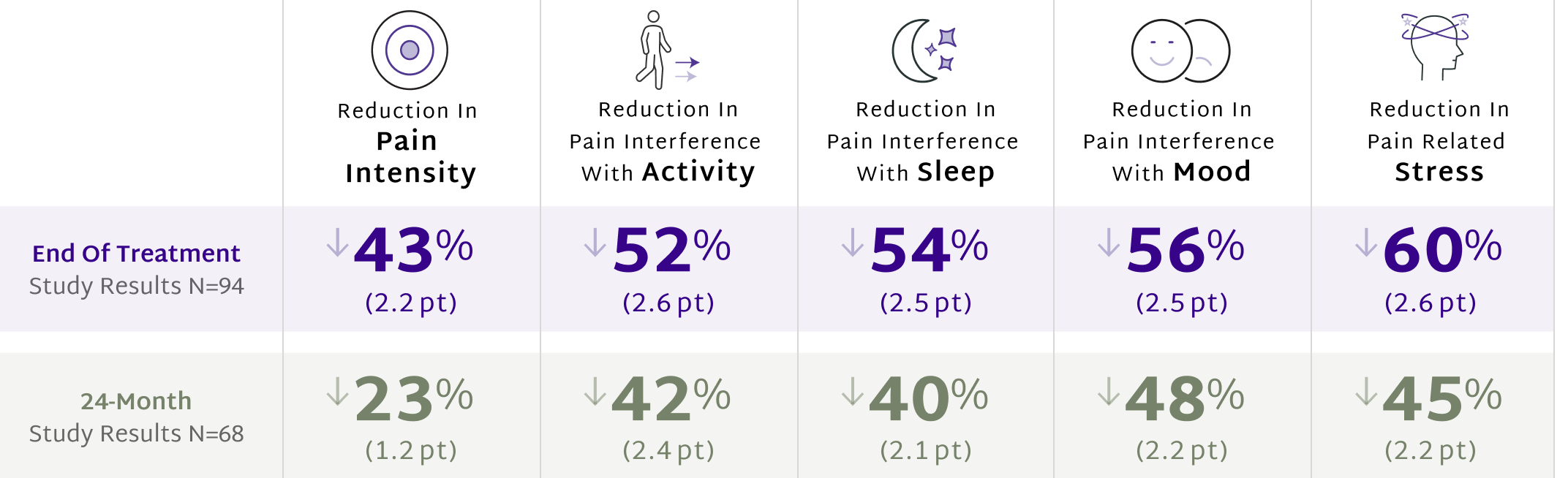
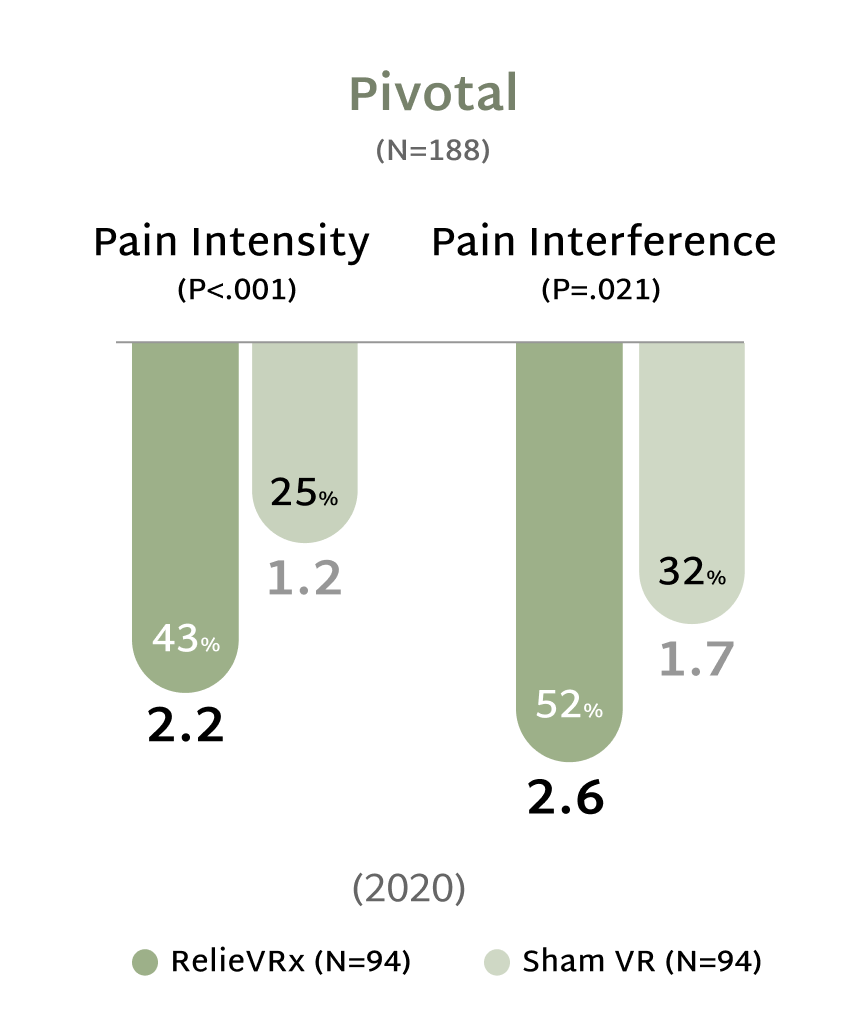
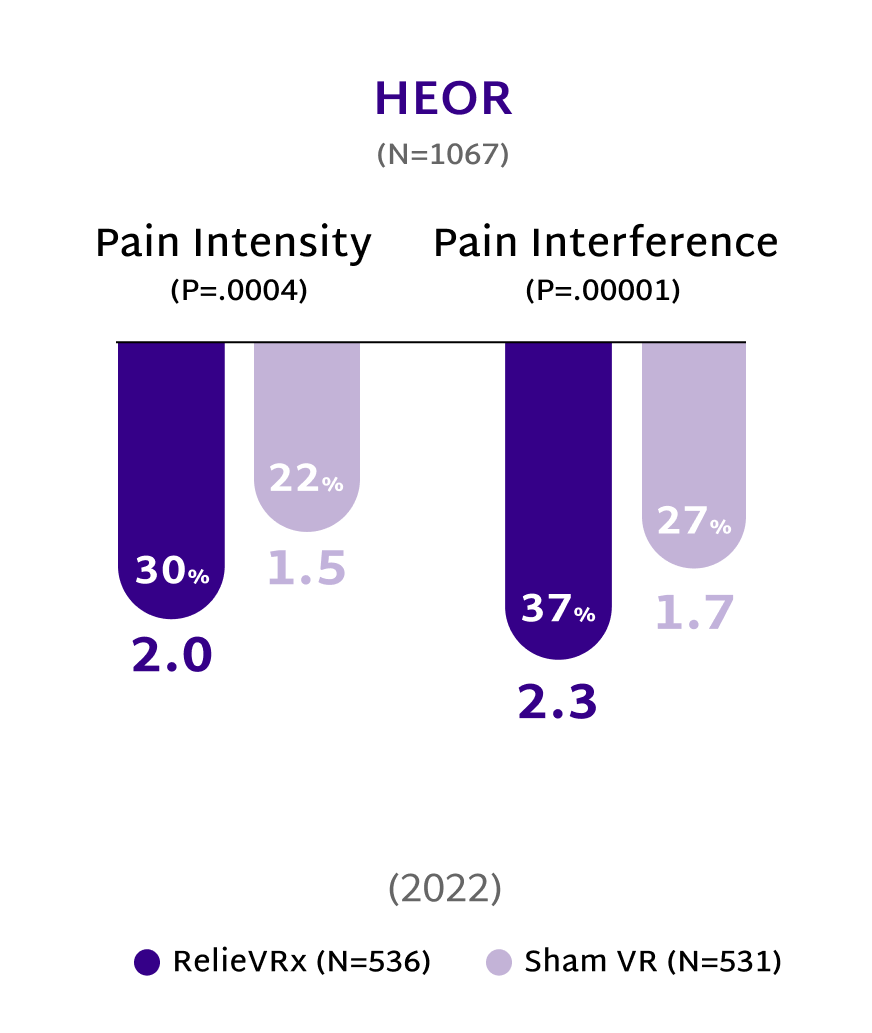
Study participants reported their pain levels on a scale from 0 to 10, using the DOD/VA Pain Scale. The values in parentheses are the mean reductions in pain ratings on this scale from baseline to the specified endpoints.
Download Clinical SnapshotPeople with CLBP are nearly 2-3 times more likely to experience moderate to severe depression, sleep disturbance, and require multiple medical modalities.4-7
In-home, self-guided therapeutic program which teaches skills and develops habits to help better control pain2
The 56-session curriculum includes:

National guidelines acknowledge the crucial role of cognitive behavioral therapy (CBT) in the management of chronic pain and recommend its utilization as a primary intervention.8

“
I find RelieVRx to be an incredibly powerful tool in the virtual reality platform because it meets all of those needs that are missed.”
- Teresa Malik-Searle, MS, PMGT-BC, ANP-BC
Give your patients the best chance at relief
Over time, chronic lower back pain treatments often lose effectiveness due to changes in the nervous system, increased psychological stress, and physical deconditioning.9-11
The RelieVRx program empowers providers to offer CBT-based pain management earlier and more effectively, alongside existing care.1,2Early intervention as an adjunctive option can support better outcomes.
Prescribe NowPatients with High Impact Chronic Pain Benefit Most From RelieVRx
Patients with High Impact Chronic Pain (HICP) experienced statistically larger pain interference reductions at End of Treatment and 12 months post treatment than those with Low Impact Chronic Pain (LICP).12
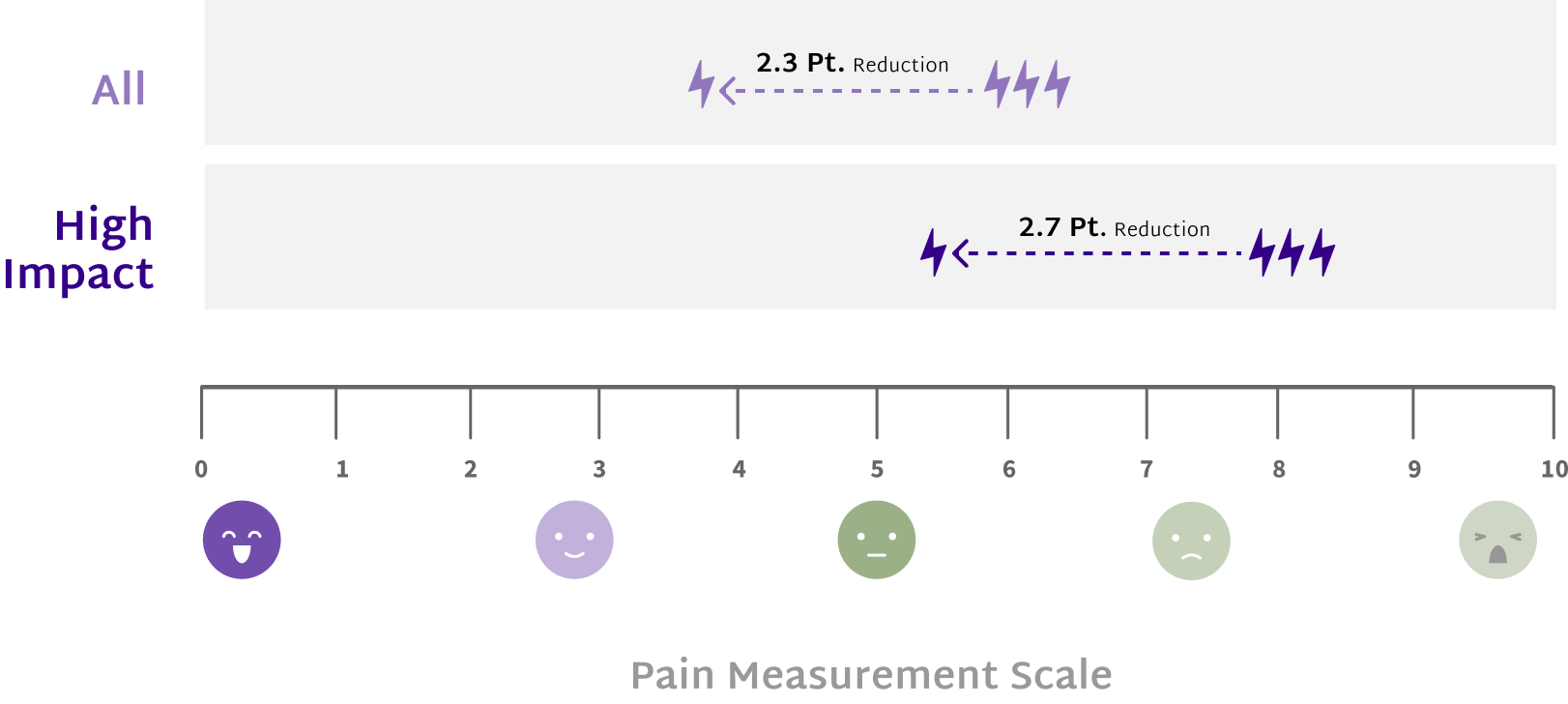
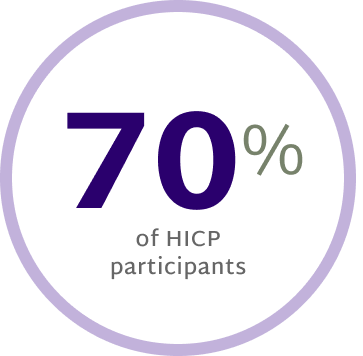
Shift from HICP at baseline to
LICP at end of treatment12
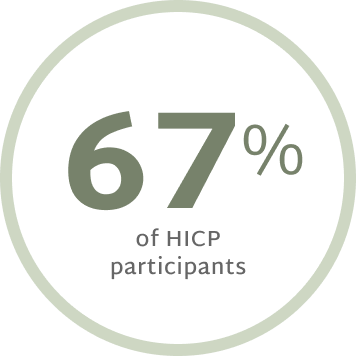
Remain LICP at 12 months
post-treatment12
Patients with HICP have higher healthcare utilization compared to those with Low Impact Chronic Pain.13
HICP is defined as chronic pain that significantly interferes with daily functioning and activities and is associated with BPI pain interference ratings ≥ 7.14
Delivers effective pain management to patients without risk of significant side effects
No Unanticipated Adverse Device Effects (UADE) or Serious Adverse Events were observed or reported in our pivotal clinical study.2
In a later study of more than 1000 participants, 1 patient reported a serious adverse event (dizziness).15
PROGRAM EXPERIENCE
Rooted in Science, Proven in Practice
biopsychosocial approach
The RelieVRx program integrates established cognitive behavioral therapy principles within an immersive environment, leading to significant and meaningful results.2
Neuroplasticicity
The sequential therapeutic program aids in altering brain areas linked to pain perception. Regular review sessions and repetition within the program result in long-lasting relief.2,3

“
You’re learning how to do that in the headset, but you’re really learning these skills so that you can apply them outside the headset as well and that's what anchors in the lasting results that we see in our studies.”
- Dr. Beth Darnall, PhD
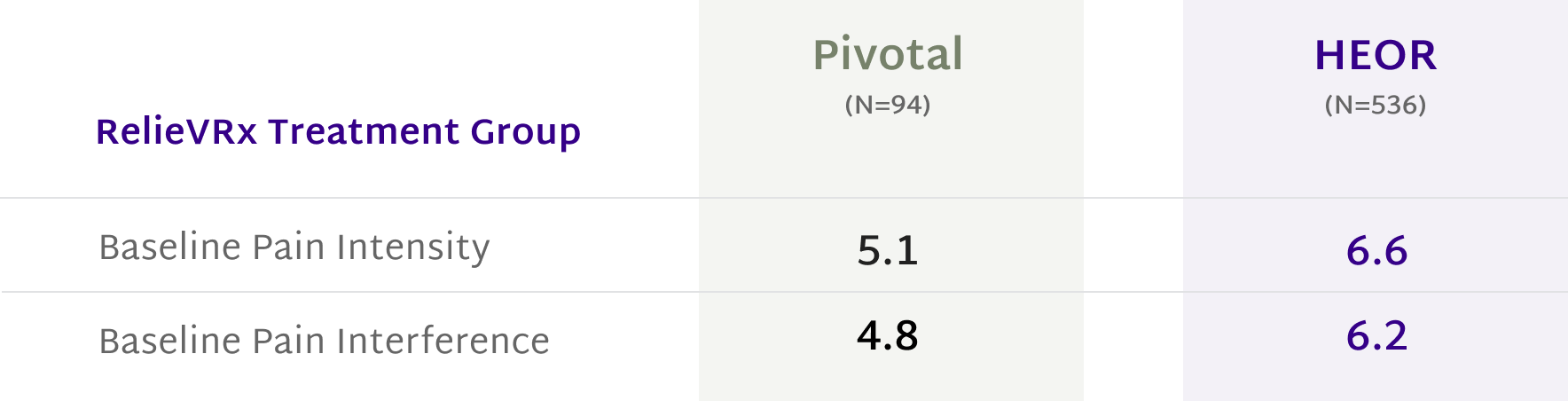
Delivers significant pain reduction with both moderate and severe chronic lower back pain
In multiple double-blinded, sham-controlled clinical trials with over 1200 patients, the RelieVRx program demonstrated clinically meaningful reductions in both pain intensity and pain interference.2,15
A reduction of 2 points or more is deemed clinically significant.
Clinical Resource Center
Explore the scientific evidence garnered from rigorous research and clinical studies.
Added to the Federal Supply Schedule in early 2023, the RelieVRx program is used nationwide by the Veterans Health Administration.
Patients are adherent to the simple, efficient, and engaging RelieVRx program.2,15
The 56 therapeutic sessions have an average duration of 6 minutes.
Easier to use than an ATM15
In-home and self-managed
Patients completed an average of 5 sessions per week
A+ satisfaction rating from users
The RelieVRx Program offers a simple user experience
The prescriber simply submits the Rx form along with the required medical documentation to the AVR Pathway support team.
The RelieVRx device is delivered directly to the patient’s home, ready to use with preloaded content
Patients will return the device in the original packaging using the provided prepaid return shipping label.
The RelieVRx program minimizes injured worker and healthcare provider challenges with traditional CBT which can include the limited availability of trained therapists, travel considerations, stigma, and cost.2
Prescribing
It’s easy to start prescribing the RelieVRx program, easy for people with CLBP to use, and easy for claims representatives to manage. Our prescription kit includes everything needed to get started. The prescriber simply submits the Rx form along with the required medical documentation to the AVR Pathway support team, and we will take care of the rest.
The AVR Pathway® team provides support for every step of the journey
The AVR Pathway team supports patients as they embark on the RelieVRx journey, serving as the primary point of contact for technical and non-medical questions.
If you are currently a RelieVRx patient and need assistance, please don't hesitate to call or email us
Indication for Use The RelieVRx program is a prescription-use immersive virtual reality system intended to provide adjunctive treatment based on cognitive behavioral therapy skills and other evidence-based behavioral methods for patients (age 18 and older) with a diagnosis of chronic lower back-pain (defined as moderate to severe pain lasting longer than three months). The device is intended for in-home use for the reduction of pain and pain interference associated with chronic lower back pain.
Contraindications
There are no known contraindications.
References
- “Device Classification under Section 513(F)(2)(De Novo).” Accessdata.fda.gov, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/denovo.cfm?id=DEN210014.
- Garcia LM, Birckhead BJ, Krishnamurthy P, Sackman J, Mackey IG, Louis RG, Salmasi V, Maddox T, Darnall BD. An 8-Week Self-Administered At-Home Behavioral Skills-Based Virtual Reality Program for Chronic Low Back Pain: Double-Blind, Randomized, Placebo-Controlled Trial Conducted During COVID-19. J Med Internet Res. 2021 Feb 22;23(2):e26292.
- Maddox T, Sparks C, Oldstone L, et al. Durable chronic low back pain reductions up to 24 months after treatment for an accessible, 8-week, in-home behavioral skills-based virtual reality program: a randomized controlled trial. Pain Med. 2023;24(10):1200-1203. doi:10.1093/pm/pnad070
- Yang H, Hurwitz EL, Li J, de Luca K, Tavares P, Green B, Haldeman S. Bidirectional Comorbid Associations between Back Pain and Major Depression in US Adults. Int J Environ Res Public Health. 2023 Feb 27;20(5):4217. doi: 10.3390/ijerph20054217. PMID: 36901226; PMCID: PMC10002070.
- Depression and Pain Comorbidity. A Literature Review. Matthew J. Bair, MD, MS; Rebecca L. Robinson, MS; Wayne Katon, MD; et al. Kurt Kroenke, MD. Author Affiliations Article Information. Arch Intern Med. 2003;163(20):2433-2445. doi:10.1001/archinte.163.20.2433
- Uchmanowicz I, Kołtuniuk A, Stępień A, Uchmanowicz B, Rosińczuk J. The influence of sleep disorders on the quality of life in patients with chronic low back pain. Scand J Caring Sci. 2019;33(1):119-127. doi:10.1111/scs.12610
- Marty M, Rozenberg S, Duplan B, Thomas P, Duquesnoy B, Allaert F; Section Rachis de la Société Française de Rhumatologie. Quality of sleep in patients with chronic low back pain: a case-control study. Eur Spine J. 2008 Jun;17(6):839-44. doi: 10.1007/s00586-008-0660-7. Epub 2008 Apr 4. PMID: 18389288; PMCID: PMC2518999.
- Ehde, D. M., Dillworth, T. M., & Turner, J. A. (2014). Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. American Psychologist, 69(2), 153-166.
- Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15.
- Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133(4):581–624.
- Verbunt JA, Seelen HA, Vlaeyen JW, van de Heijden GJ, Heuts PH, Pons K, Knottnerus JA. Disuse and deconditioning in chronic low back pain: Concepts and hypotheses on contributing mechanisms. Eur J Pain. 2003;7(1):9–21.
- Maddox T, Sparks C, Oldstone L, et al. Durable chronic low back pain reductions up to 24 months after treatment for an accessible, 8-week, in-home behavioral skills-based virtual reality program: a randomized controlled trial. Pain Med. 2023;24(10):1200-1203. doi:10.1093/pm/pnad070
- Herman PM, Broten N, Lavelle TA, Sorbero ME, Coulter ID. Health Care Costs and Opioid Use Associated With High-impact Chronic Spinal Pain in the United States. Spine (Phila Pa 1976). 2019 Aug 15;44(16):1154-1161.
- Cook, K., You, D., Korff, M. et al. Defining chronic pain impact levels: a patient-clinician approach using PROMIS® pain interference scores. J Patient Rep Outcomes 9, 103 (2025). https://doi.org/10.1186/s41687-025-00908-y
- Maddox, T., Oldstone, L., Sparks, C., Sackman, J., Oyao, A., Garcia, L., Maddox, R., Ffrench, K., Garcia, H., Irvin, A., Maislin, D., Keenan, B., Bonakdar, R., & Darnall, BD (2023). At-home virtual reality program for chronic lower back pain: A randomized sham-controlled effectiveness trial in a clinically severe and diverse sample. Mayo Clinic Proceedings: Digital Medicine, 2023;1(4):563-573.